1820-1940
Early Work Toward Harmonization
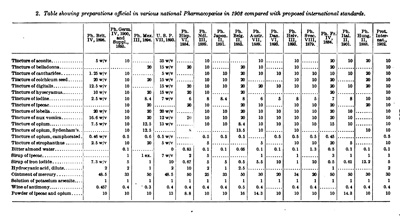
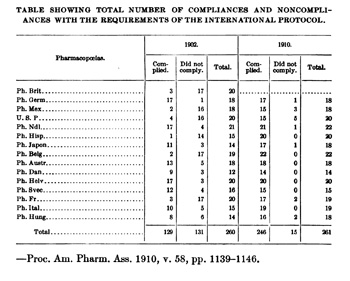
The struggle to develop harmonized pharmacopoeial standards began with the First International Pharmaceutical Congress in 1865. By the Second International Congress in 1867, the creation of an international pharmacopoeia and uniform standards for medicines had become its main objectives. Although the International Pharmaceutical Congresses continued to meet regularly throughout the nineteenth century, they made little progress, due to variations between national pharmacopeias, communication difficulties, and the broad scope of topics discussed at the Congresses. In the wake of failure to establish any international standards, a 1902 conference in Brussels was organized with the sole focus of securing international uniformity for the strength of 22 drugs. With its limited scope and wide representation of pharmacopoeial bodies, including USP's President Dr. Horatio Wood, the meeting was a success and led to the ratification of the Agreement for the Unification of the Formulae of Potent Drugs in 1906. By the publication of the next USP in 1910, all but one of the heroic medicines discussed at the conference had been revised to comply with the new "Brussels Protocol." Following the success of the Brussels conference, several attempts were made to establish a permanent organization to aid in the creation of uniform standards among pharmacopeias, including the International Bureau of Information in 1913, and the International Pharmaceutical Federation in 1923, both of which were supported financially and in spirit by USP but failed to gain international support.