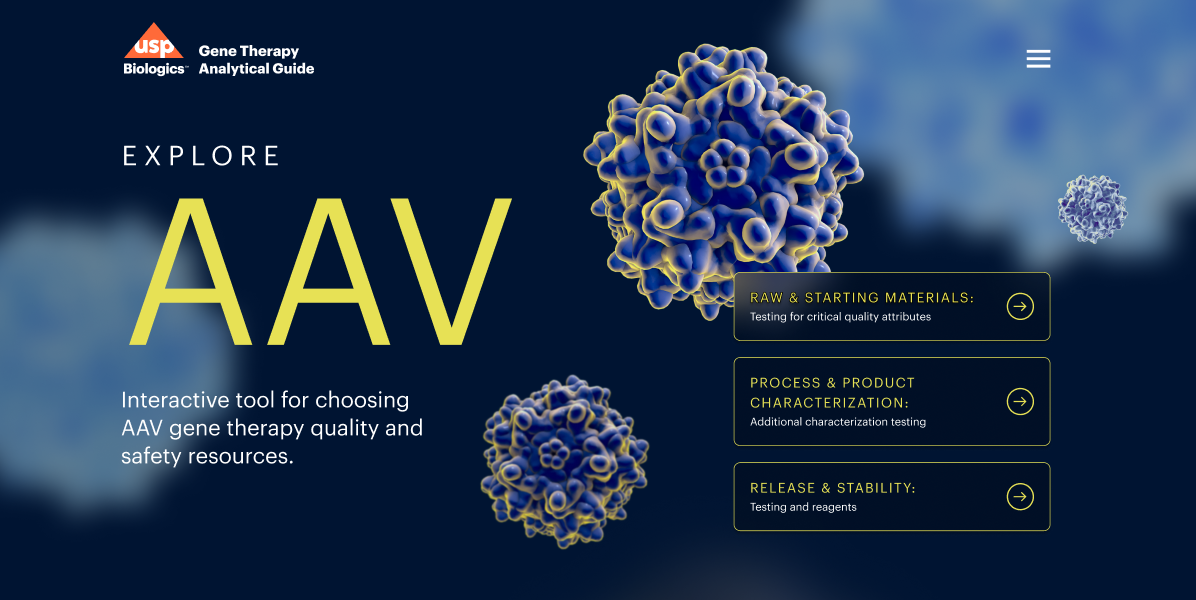
Emerging biologics are advancing rapidly, and manufacturers are faced with the complex challenge of ensuring their purity and quality to avoid development and commercial disruptions, costly setbacks and jeopardizing public health.
USP Biologics is prioritizing the ongoing development of state-of-the-art analytical tools, standards and solutions to support regulatory predictability, allowing manufacturers to operate with a high level of confidence and certainty throughout the drug development and approval process across a variety of modalities.
USP Biologics journey towards innovation involves setting higher standards, embracing advanced technologies, providing tailored support, and fostering collaboration. These efforts collectively benefit both manufacturers, who are equipped to meet the evolving demands of the biologics industry, and populations who can trust the quality of their treatments. USP Biologics is your partner for solutions that empower your success in many ways:
- Stringent Standards and Materials: USP has continuously created solutions for measuring and maintaining biologics quality standards, ensuring the global supply chain delivers safe and effective treatments and that manufacturers are guided by rigorous, fit for purpose methodology.
- Complete Quality Ecosystem: USP aims to support the development and manufacturing of biotherapies throughout the product lifecycle, including standards for cell line development through fill finish.
- Adaptation to New Technologies: USP remains at the forefront of adopting and incorporating new technologies into our standards and guidelines to enable faster and more accurate quality assessments, reducing development and manufacturing time and costs.
- Support: USP provides manufacturers with invaluable technical support and best practices, helping them navigate complex regulatory landscapes. By partnering with USP, biologics manufacturers can confidently assess regulatory requirements, reducing delays in product approval and market access.
- Education and Training: USP invests in education and training programs that deliver the latest knowledge and best practices in biologics production. This continuous learning helps build global regulatory and manufacturing capability.
- Collaborative Partnerships: USP fosters collaborative partnerships with industry stakeholders, promoting knowledge exchange and fostering an environment of continuous improvement.
Read more