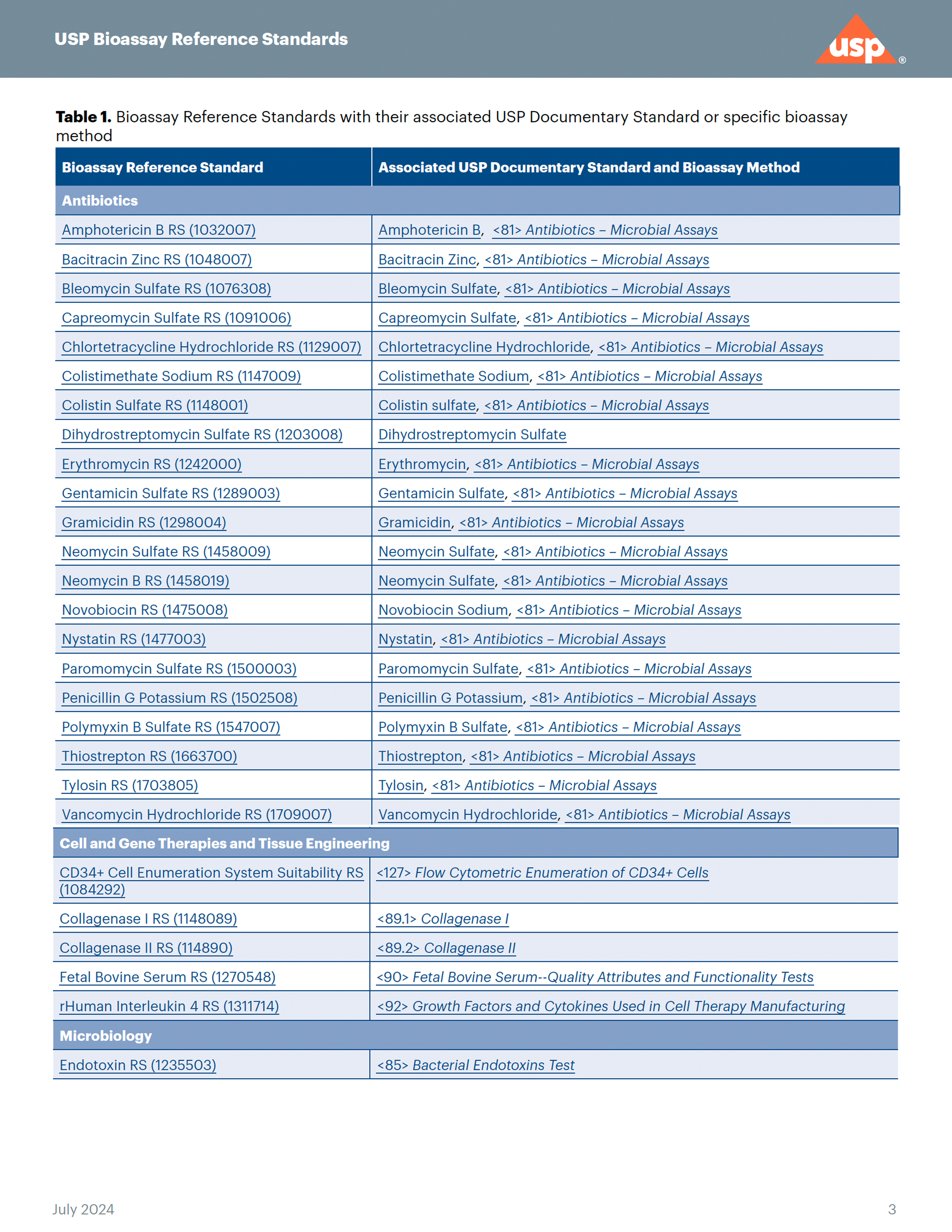
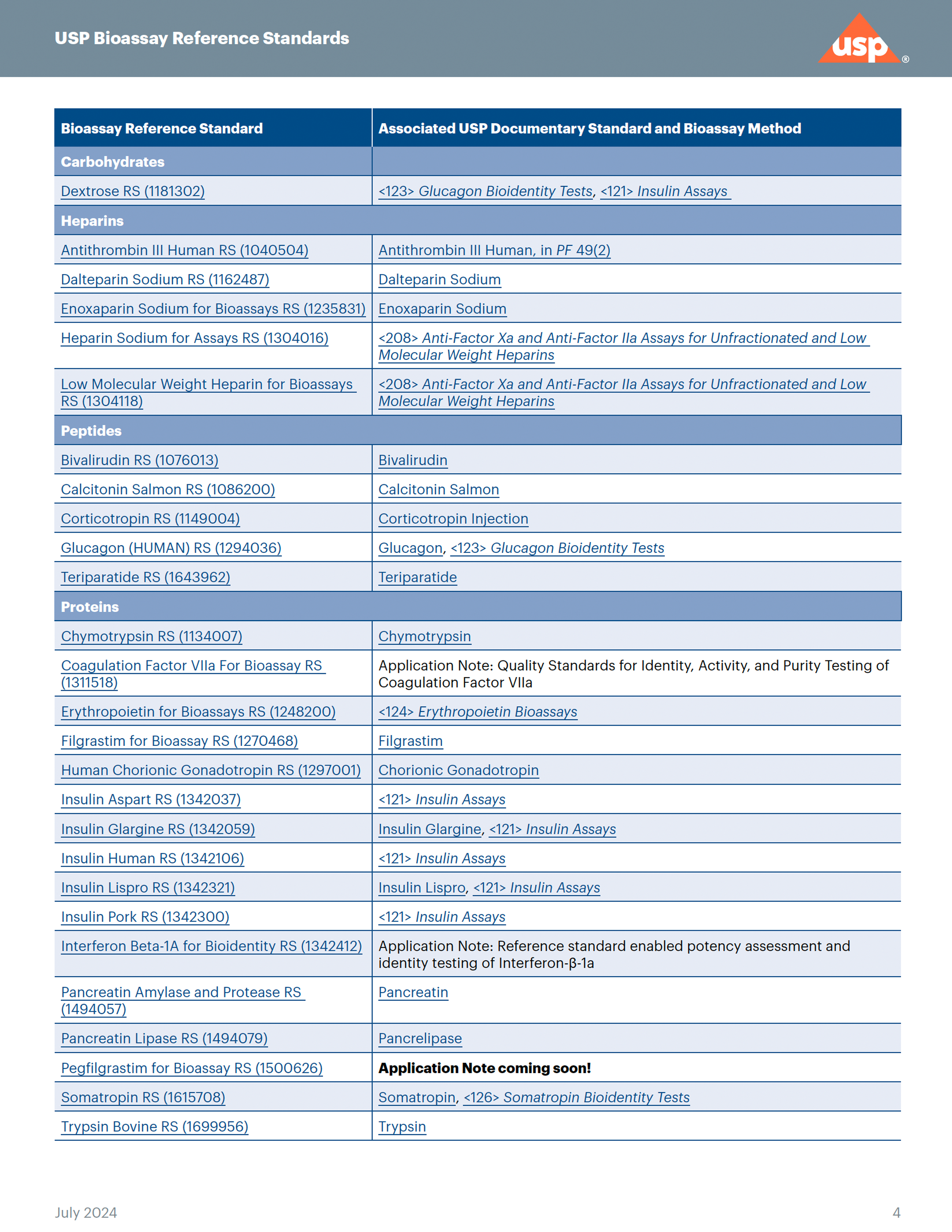
Enabling consistent potency measurements over a drug's lifetime
Biological assays (also called bioassays) are an integral part of the quality assessment required for the manufacturing and marketing of many biological and some non-biological drug products and are often used to assign potency. Bioassays quantify a drug’s ability to modify a biological process, providing insights into its mechanism of action. Bioassays are inherently variable because they rely on biological materials (either the biologically derived drug or the biological substrate [e.g., animals, living cells, or functional complexes of target receptors] on which it might act) that can change over time, thus impacting assay results.
Among many reasons, they can be impacted by:
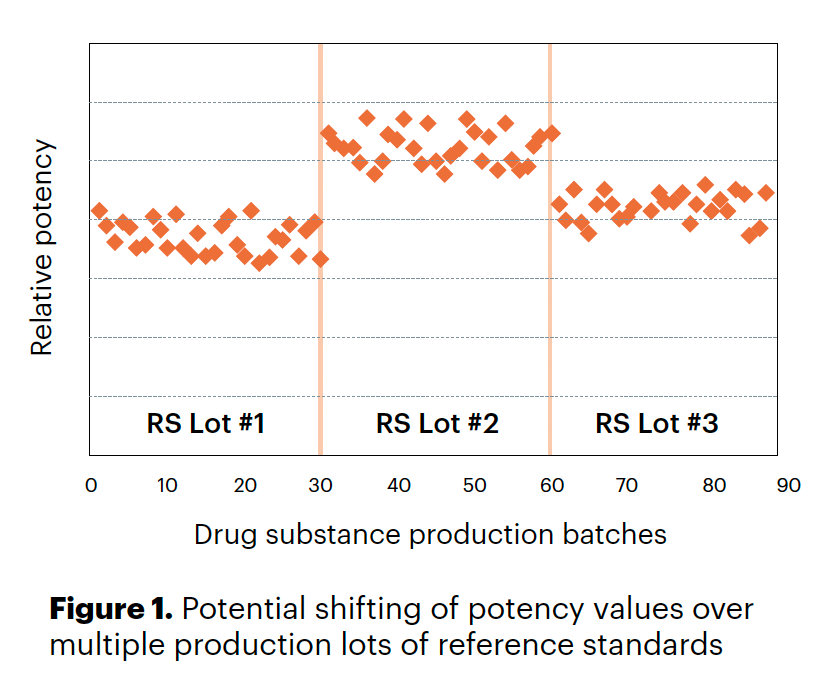
- shifts in potency when using different lots of in house standards (Figure 1); or
- drifts in the potency assignment due to degradation of the drug product or reference standard material (Figure 2); or
- new lots of critical reagents, new instruments, etc. which can cause shifts in results (Figure 1).
Once a bioassay is implemented for a drug, it is important to monitor its behavior over time. It is imperative to have and use a drug-specific reference standard that has a consistent value that can be used to compare different drug manufacturing lots.