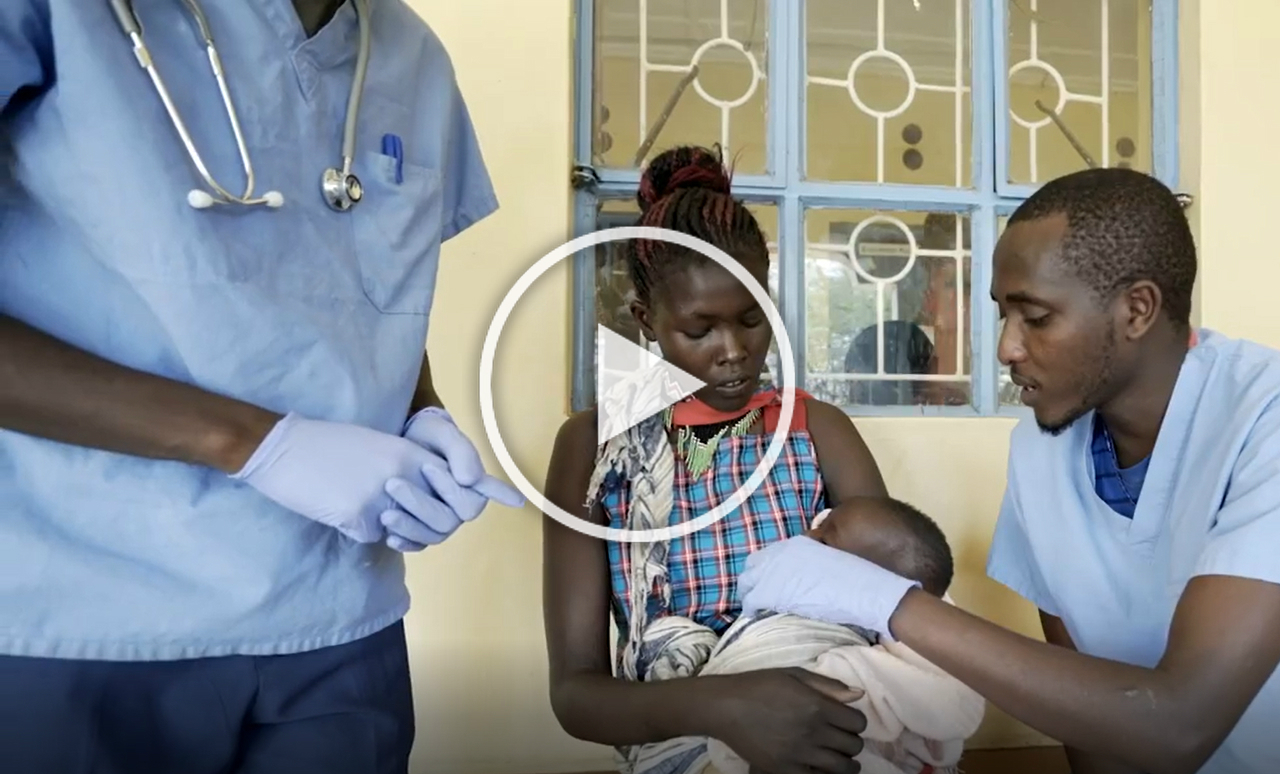
Protecting mothers and babies
More women and babies are surviving childbirth than ever before, news worth celebrating. Even so, approximately 2.8 million mothers and infants die from complications associated with pregnancy and childbirth annually, most in low-income countries. Severe bleeding, high blood pressure during pregnancy and infections account for most fatalities, many of which are preventable. Poor-quality medicines used to treat these conditions, contribute to the high number of preventable maternal and infant deaths.
More than 20% of drugs on the WHO Model List of Essential Medicines lack pharmacopeial standards, which is dangerous. Without a monograph to articulate the quality expectations for a medicine and the tests to validate that a medicine and its ingredients meet these criteria, a medicine's identity, strength and purity cannot be quality assured. In addition, robust regulatory systems are required to ensure that only quality medicines enter a country's supply chain and that quality is protected as the medicines move through the country.
Mothers and babies deserve access to medicines they can trust
Since 1820, USP has created public quality standards and programs to ensure the safety and benefit of medicines. In 2017, we established the Global Health Standards program to ensure that monographs and reference standards exist for essential medicines that may not be legally marketed in the United States, but which have been approved by a stringent regulatory authority in other parts of the world.
One such medicine is chlorhexidine gel. WHO recommends the medicine to prevent infections in newborns in areas with high rates of home births and neonatal mortality. To stimulate the medicine's production in countries including Ethiopia, Nigeria, and Pakistan, the Promoting the Quality of Medicines (PQM) program, funded by USAID and implemented by USP, collaborated with the Chlorhexidine Working Group to advance a strategy for local production and regional distribution. As part of this strategy, USP developed and shared a standard for this lifesaving medicine to allow manufacturers and regulators to better evaluate its quality. USP also provided technical assistance to several local manufacturers, which were able to attain national regulatory approval and increase the supply of locally produced quality-assured chlorhexidine.
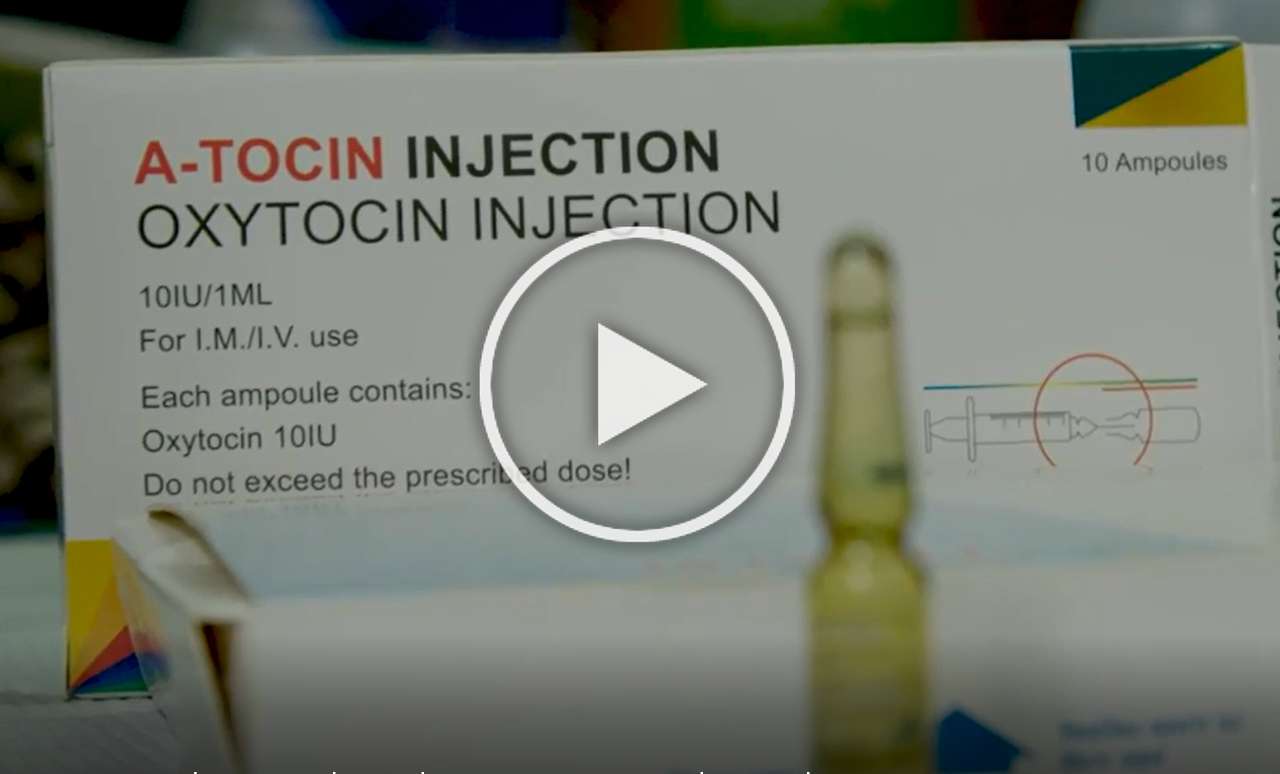
WHO also recommends the use of oxytocin to prevent and manage postpartum hemorrhage and the use of magnesium sulfate to prevent and treat pre-eclampsia and eclampsia, two leading causes of maternal death. In Nigeria, with PQM and other partners, USP has strengthened good manufacturing practices for local manufacturers of both medicines, targeting the country's high maternal mortality rate by offering greater patient access to these lifesaving medicines.