Trust in healthcare
Trust is earned, not given. It's built over time by consistently demonstrating traits like honesty, competence, selflessness and reliability. Trust can be broken by a single incident or erode over time. And when trust affects something as foundational as your health, people and institutions must consistently demonstrate that they are worthy of it. For 200 years, the U.S. Pharmacopeia (USP) has been dedicated to improving and protecting the public's health by building trust in the quality of medicines.
The current state of trust in healthcare
Over the last two centuries, science and medicine have made enormous strides in the ability to diagnose and treat disease. Most healthcare professionals are ethical and committed to the wellbeing of their patients. But as systems for patient care and drug manufacturing have become increasingly complex, there are more and more opportunities for something to go wrong, quality to be compromised and trust to be damaged.
Something went wrong…
Antibiotics have been widely used to treat bacterial infections since the 1940s. Recently, physicians became concerned when they began seeing a sharp increase in patients with allergic reactions after receiving an injection of the antibiotic, gentamicin, used to treat serious bacterial infections. Symptoms included flushing, itching, hives, shortness of breath, decreased blood pressure and increased heart rate. Regulatory agencies and healthcare professionals scrambled to identify the cause; Canada, the United Kingdom and Australia all recalled batches of gentamicin and issued regulatory warnings.
Public health officials found elevated histamine levels in gentamicin, which led to the allergic reactions patients were experiencing. Some batches of gentamicin had histamine levels 7-fold higher than the threshold at which allergic reactions start to appear. The elevated histamine levels were caused by improper storage conditions for one of the raw materials used in the process for manufacturing the antibiotic.
When trust is broken
Over the past 70 years, antibiotic use has become increasingly common. The quality and safety of antibiotics is often taken for granted. They are trusted to help make patients well but, in the case of these gentamicin injections, that didn't happen.
Patients and physicians depend on antibiotics and other drug products to be what they are purported to be. Patients trust that what they receive from the pharmacist contains the correct active ingredients in the correct amounts, that the inactive ingredients function as intended and that they don't contain other harmful substances.
Whether the cause is deliberate or accidental, incidents in which poor-quality medicines result in illness and death have had a devastating impact not only on the people directly affected, but also in the public's trust of healthcare providers, pharmaceutical companies and the global supply chain. This lack of trust then directly impacts public health, with fewer people seeking treatment or complying with treatment regimens.
Safeguarding medicine quality
Quality control and quality assurance programs help pharmaceutical manufacturers and regulatory agencies around the world supply safe, quality drug products for patients. One component of the systems that monitor quality involves the use of public quality standards. These testing methods and acceptance criteria, along with physical specimens of substances, provide benchmarks against which manufacturers and regulators can measure the quality of their processes and products.
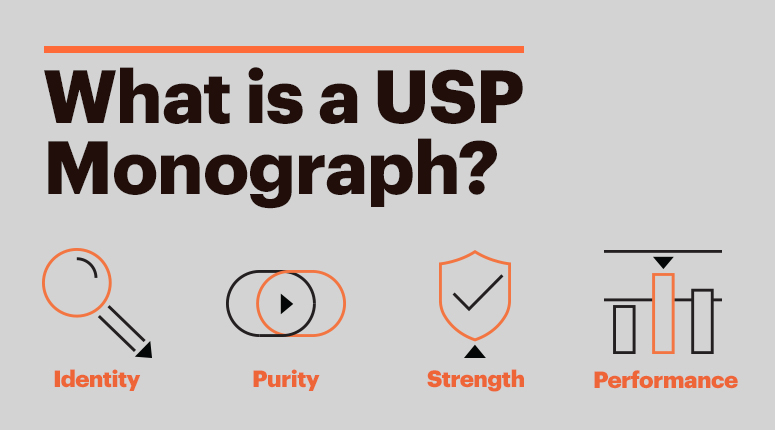
U.S. law mandates that all drug products sold in the United States must meet the standards established by the U.S. Pharmacopeia for identity, purity, strength, performance and other quality specifications for proper storage, distribution, packaging and labeling. USP standards are trusted in 140 countries around the world to help ensure the quality of medicines.
Standards and continuous revision
For 200 years, USP has been committed to protecting patient safety through public quality standards and programs that educate stakeholders on the proper use of standards. As scientific discoveries and technical advances in medicine have occurred, USP has kept pace. With the constant transformation that occurs in health and medicine, we will continue to face new unanticipated challenges. USP's commitment to continuous revision means the organization can respond to new challenges. Case in point: a standard that will include validated methods to quantitate histamine in antibiotics is currently in development, which will enable manufacturers to evaluate histamine levels to better ensure the quality and safety of gentamicin, preventing further adverse reactions.
But gentamicin isn't the only antibiotic that uses the raw materials that were linked to the allergic reactions in its manufacturing process. Other antibiotics could potentially harbor elevated levels of histamines and cause adverse reactions in patients, making a standard all the more important. Test methods for other antibiotics will also be developed and added to the quality standard as they become available.
 Experts from the pharmaceutical industry, academia, regulatory agencies and other organizations around the world volunteer their time to collaborate with scientists at USP in developing standards for monitoring the quality of medicines. As part of the development process, these standards go through a public vetting period, during which all interested parties can provide input and feedback on the standard being proposed. This open and transparent process ensures that stakeholders from around the world have an opportunity to weigh in, a multi-disciplinary approach that relies on diversity of perspectives to develop standards that health practitioners, governments, industry and others have trusted for 200 years. According to Dr. Jaap Venema, chief science officer at USP, "USP's volunteers share their collective brainpower that enables us to draw on the best science in order to produce the best standards."
Experts from the pharmaceutical industry, academia, regulatory agencies and other organizations around the world volunteer their time to collaborate with scientists at USP in developing standards for monitoring the quality of medicines. As part of the development process, these standards go through a public vetting period, during which all interested parties can provide input and feedback on the standard being proposed. This open and transparent process ensures that stakeholders from around the world have an opportunity to weigh in, a multi-disciplinary approach that relies on diversity of perspectives to develop standards that health practitioners, governments, industry and others have trusted for 200 years. According to Dr. Jaap Venema, chief science officer at USP, "USP's volunteers share their collective brainpower that enables us to draw on the best science in order to produce the best standards."
The impact of trust in healthcare
Trust can impact a patient's health. One study found that two-thirds of patients with high levels of trust always take their medications while only 14% of those with low levels do. Lack of trust in healthcare has personal, financial and social implications. If patients don't seek the medical care they need, the quality and possibly the length of their life may be reduced. Failure to properly treat a medical condition can lead to the patient's condition getting worse, which can result in lengthier, more intensive and more expensive treatment.
Building the public's trust in the healthcare system is complex. From relationships with health providers, to the quality of interactions and experiences at health facilities, to the therapeutic benefits of medicines and in many other areas, there are ample opportunities to either build trust or to watch it erode. When it comes to trust in medicines, for 200 years USP has been quietly laying the foundation for safety and quality that is essential to giving more patients access to the medicines they need to live longer and healthier.