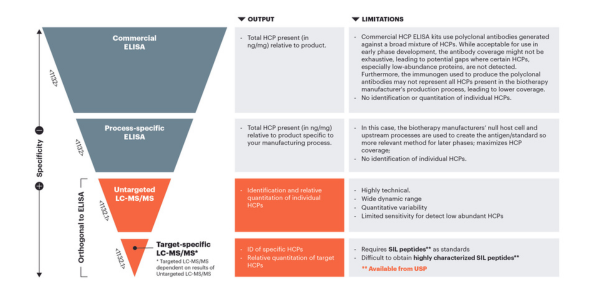
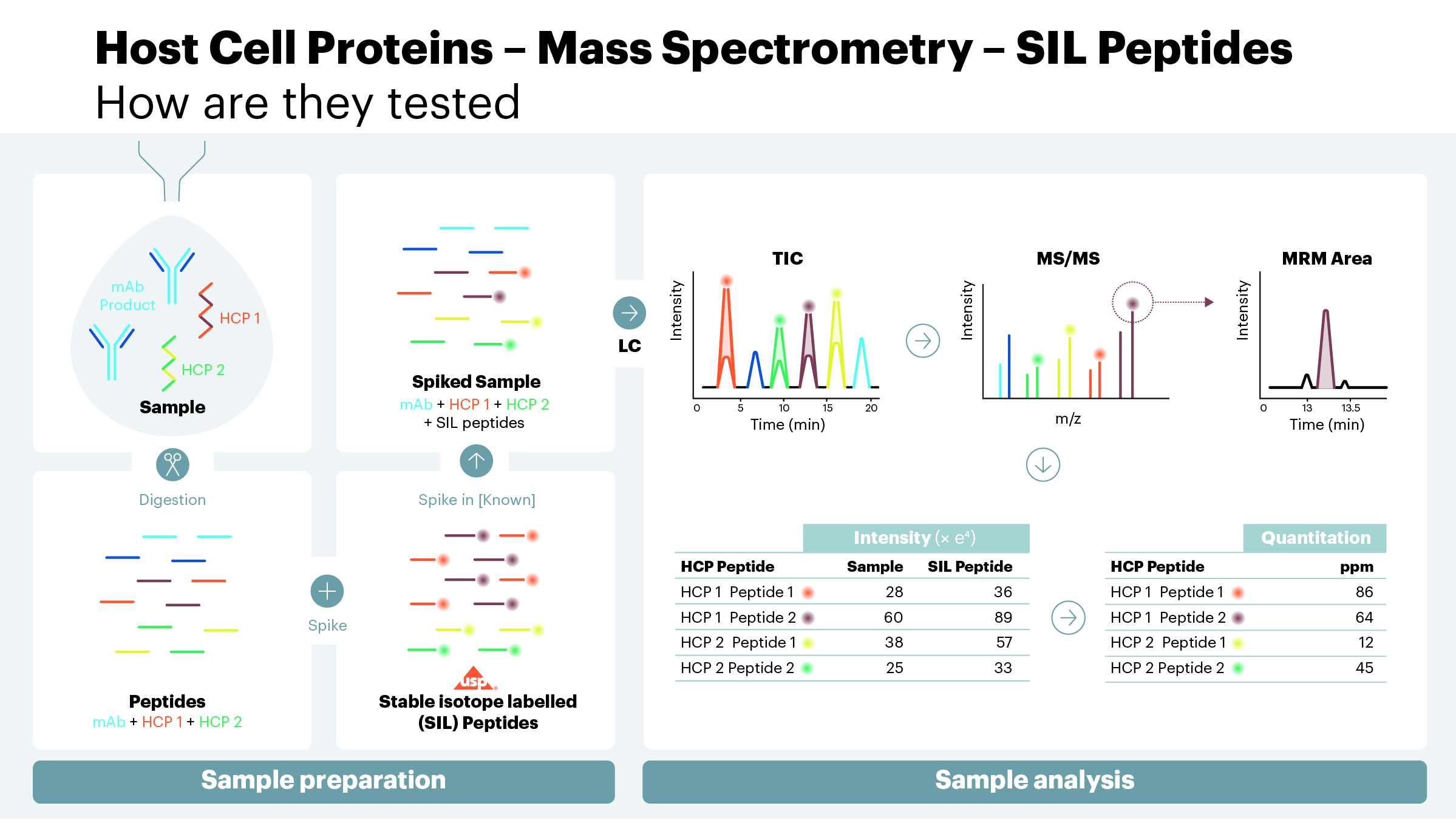
Host cell proteins (HCPs) are process-related impurities produced by the host organism during biotherapeutic manufacturing. During the purification process, a majority of HCPs are removed from the final product. Residual HCPs that copurify with the manufactured antibody therapy, even at a low level, can elicit immune responses in patients. HCPs can also directly effect the quality of the drug product itself. For example, proteolytic HCPs, even in minute quantities, can cleave the desired protein product over time, reducing or eliminating biological potency or altering stability.
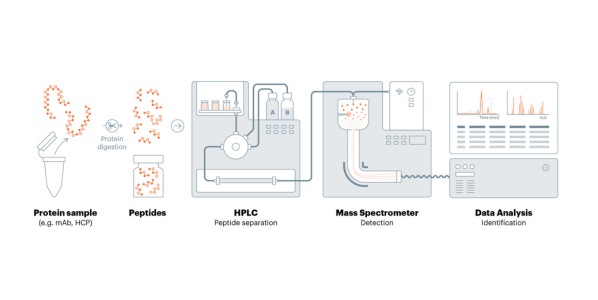
The measurement and control of these HCPs are important to achieve the desired efficacy, shelf-life, or other important attributes of the therapy. USP recently released a draft of <1132.1> Residual Host Cell Protein Measurement in Biopharmaceuticals by Mass Spectrometry as a new general chapter to introduce mass spectrometry (MS) techniques for identification and quantitation of HCPs lingering in recombinant therapeutic products. These evolving methods for HCP analysis are orthogonal to ELISA and other analytical methods and enable the user to build an understanding of HCP profiles and clearance patterns throughout the process. To view the chapter visit here.