2010-future
Continued Growth:
Sites & Expertise
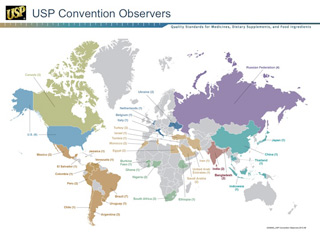
To better support international standards and programs, USP continues to expand its facilities and expertise base. In 2011, USP completed a 100,000-square-foot state-of-the-art laboratory and office expansion at its USP–India site. A similar expansion is planned for USP–China. USP envisions the enhanced sites to be "Centers of Excellence" for standards development, verification, and education activities. India's Center of Excellence will focus on medicines, and China's on food. To guide global standards-setting efforts, USP created new Expert Committees (ECs) for the Medicines Compendium (MC) in South Asia, East Asia, and Latin America for chemical medicines. USP also created an international Expert Committee on Biologics for the MC. In addition, more than 200 volunteer experts from 35 countries serve on USP's ECs and Expert Panels.